
ALL CATEGORIES
COMPANY INFO
Tunable Diode Laser Absorption Spectroscopy solution
TDLAS working theory
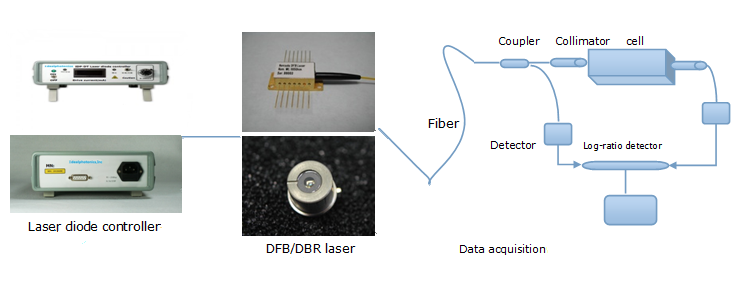 Product Solutions and Laser
diode controller
Product Solutions and Laser
diode controllerOutput of high stable drive current(up to 32A)
High precision temperature control±0.2℃
Safe boot with multiple protection
High power control
IDP DFB/DBR laser

14 PIN Butterfly package:wavelength760~2200nm
5.6 TO Package wavelength from1280to 3270nm
wavelength:2465nm,2475nm,2485nm,2495nm,2505nm,3270nm are all available from Idealphotonics Inc.
Output power:5mW, 10mW,20mW ,30Mw
Coupler or Collimator
Wavelength:460~2000nm
Configure(For coupler):1*2,2*2,3*3.Output fiber:PM,SM,MM
Find more in laser components
Detector
We can provide you different materials including Silicon detector,InGaAs detector.Please contact our sales for further information. Email:info@idealphotonics.com
Typical Absorption Spectroscopy
O2 Absorption Spectroscopy
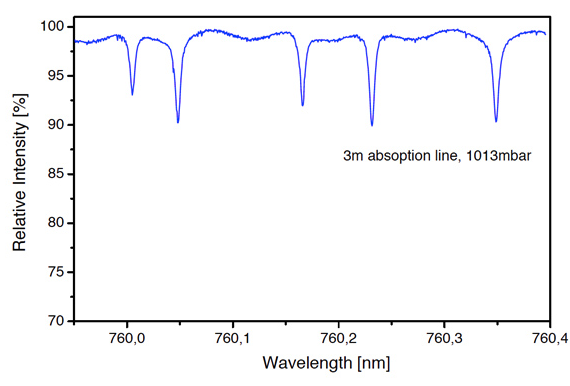
Oxigen is the most important molecule on earth. Without oxigen, there would be no life possible on this planet. In combustion processes, the amount of oxigen needs to be exactly measured. In the Hitran database, many transitions can be found, and there are several strong absorption lines the 760nm regime, for example. Some of these strong line at 760nm are shown in the figure, detected with a simple oxigen absorption experiment.
Caesium Absorption Spectroscopy
Caesium is a very reactive alcali metal of the first group of the table of elements. It was first discovered by Bunsen and Kirchhoff in 1860 using spectroscopic investigations. It is characterized by one valence electron.
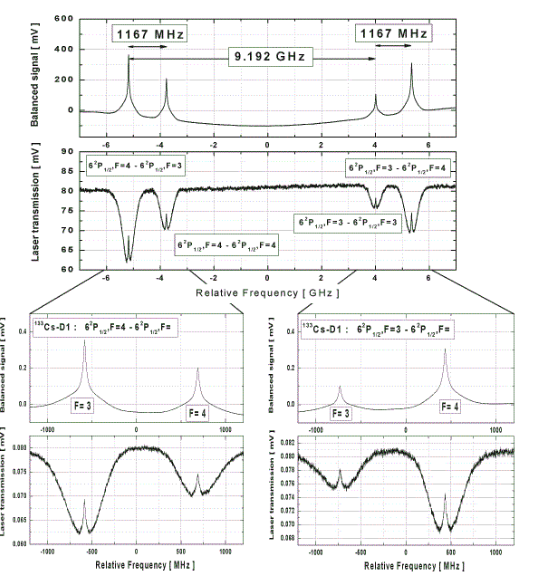 The
spectra show the saturated absoption of the D1 line of Caesium at
895nm. The spectra are detected with a continuous scan with a Sacher
Lasertechnik tunable diode laser system
The
spectra show the saturated absoption of the D1 line of Caesium at
895nm. The spectra are detected with a continuous scan with a Sacher
Lasertechnik tunable diode laser system
CH4 Absorption Spectroscopy
Methane is one of the most important organic
molecule on earth. However, in some 'applications' the amount of methane needs
to be exactly measured. In the Hitran database, many transitions can be found,
and there are several strong absorption lines the 1655nm regime, for example.
Some of these strong line in the 1655nm regime are shown in the figure,
detected with a simple absorptionexperiment 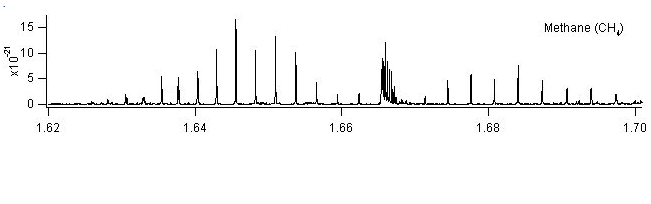
HCl Absorption Spectroscopy
Hydrogen Chloride is one of the most important
acits. However, in some 'applications' the amount of hydrogen chloride needs to
be exactly measured. In the Hitran database, several transitions can be found.
A popular transition is within the first vibrational overtone around 1742nm.
Potassium Absorption
Spectroscopy
Potassium is a very reactive alcali metal of the
first group of the table of elements. It was first discovered by Humphry Davy
in 1807 when potassium and sodium were individually isolated from different
salts by electrolysis. It is characterized by one valence electron.
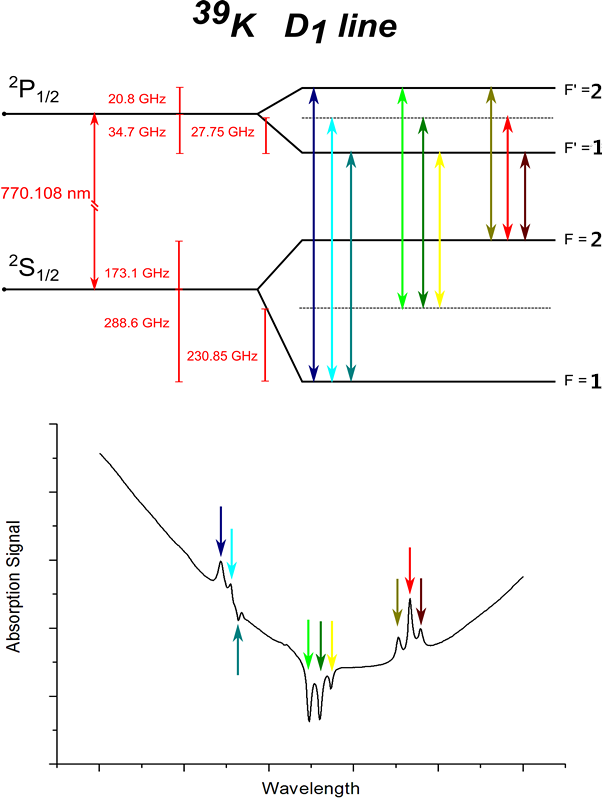
The
spectra show the saturated absoption of the D1 line of Potassium at 770nm. The
spectra are detected with a continuous scan with a Sacher Lasertechnik tunable
Littman/Metcalf diode laser system.
Water Vapor Spectroscopy(H2O 1392nm)
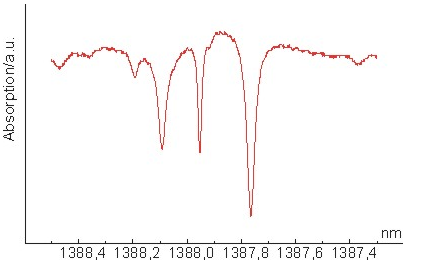
Water is the most important molecule on earth.
Without water, there would be no life possible on this planet. However, in some
'applications' the amount of water vapor needs to be exactly measured. In the
Hitran database, many transitions can be found, and there are several strong
absorption lines the 1390nm regime, for example. Some of these strong line at
1388nm are shown in the figure, detected with a simple water vapor absorption
experiment
Rubidium Absorption Spectroscopy
Rubidium is a very reactive
alcali metal of the first group of the table of elements. It was first
discovered by Bunsen and Kirchhoff in 1861 using spectroscopic investigations.
Due to its typical red color of the spectral lines it was named after the Latin
word 'rubidus' (darkred). It is characterized by one valence electron. The
natural abundance of Rubidium isotopes is 85Rb(72.2%) and 87Rb(27.8%).
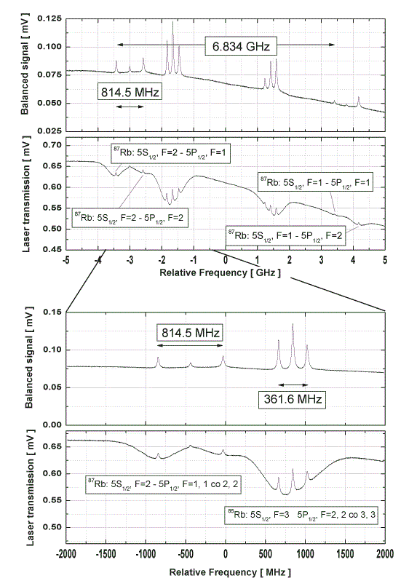
The
spectra show the saturated absoption of the D2 line of Rubidium at
795nm. The spectra are detected via one continuous scan with a Idealphotonics
tunable diode laser system.










 编辑
编辑